

| Model | Chamber Volume (L) | Internal Dimensions (W × D × H, mm) | DIN 1/1 (480×250 mm) | DIN 1/2 (480×125 mm) | Typical Use Case |
|---|---|---|---|---|---|
| VS-35R | 35 L | 320 × 250 × 450 | 1 | 2–3 | Dental, labs |
| VS-50R | 50 L | 350 × 300 × 475 | 2 | 4 | Clinics, mobile units |
| VS-75R | 75 L | 400 × 350 × 530 | 3 | 6 | Outpatient centers |
| VS-100R | 100 L | 450 × 370 × 600 | 4 | 8 | OR prep, CSSDs |
| VS-120R | 120 L | 450 × 400 × 670 | 5 | 10 | Private hospitals |
| VS-150R | 150 L | 500 × 420 × 720 | 6 | 12 | Hospital ORs |
| VS-200R | 200 L | 550 × 450 × 800 | 8 | 16 | CSSDs, day clinics |
| VS-250R | 250 L | 600 × 470 × 880 | 10 | 20 | Multi-OR facilities |
| VS-300R | 300 L | 650 × 500 × 900 | 12 | 24 | Regional hospitals |
| VS-400R | 400 L | 700 × 500 × 1150 | 14 | 28 | Large CSSDs |
| VS-500R | 500 L | 750 × 550 × 1200 | 16 | 32 | Medical centers |
| VS-600R | 600 L | 800 × 550 × 1350 | 20 | 40 | Tertiary hospitals |
| VS-750R | 750 L | 850 × 600 × 1450 | 24 | 48 | Sterilization hubs |
| VS-1000R | 1000 L | 900 × 650 × 1700 | 30–32 | 60–64 | National-level CSSDs |

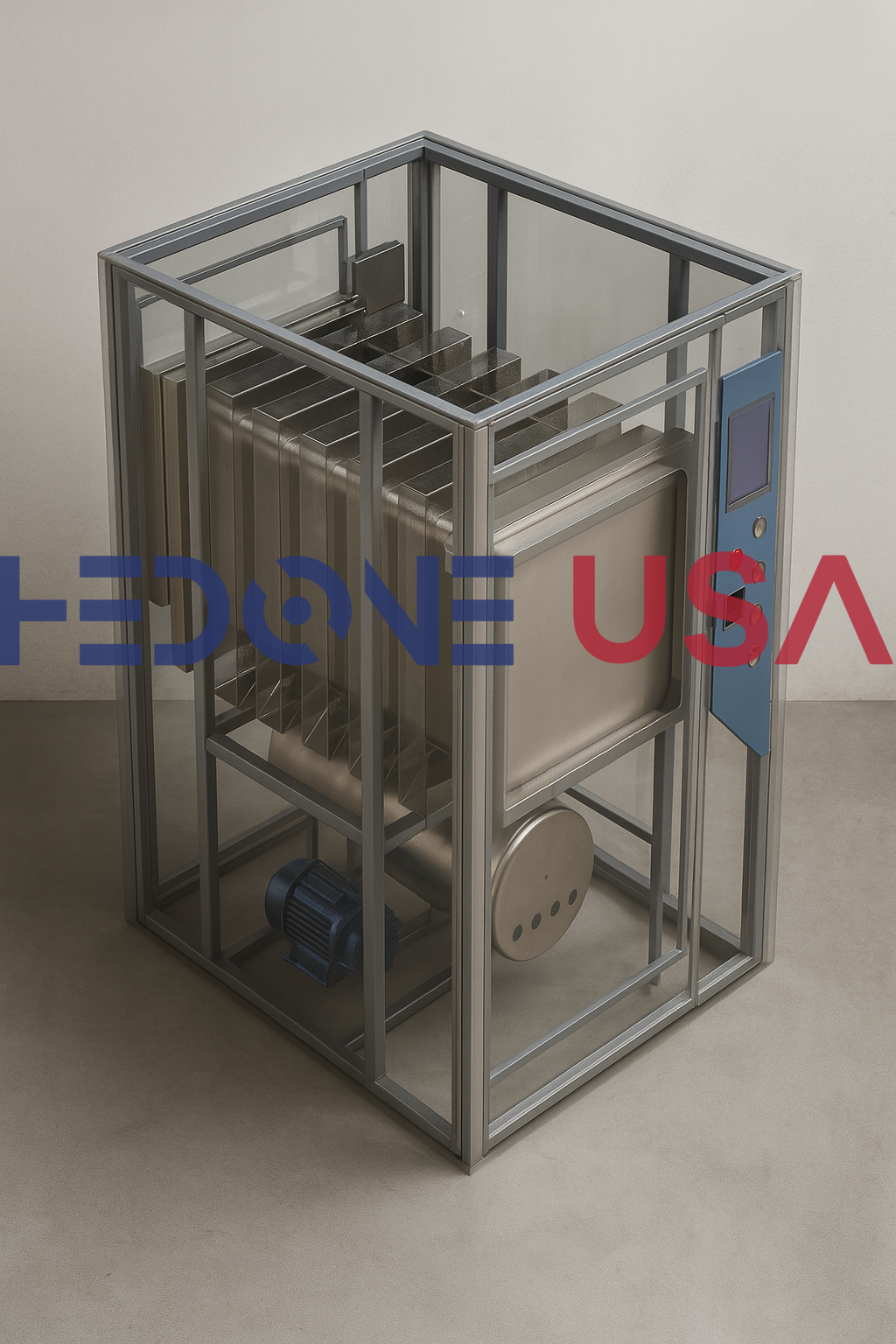
Chamber Construction: High-grade SUS 304 or SUS 316L stainless steel, polished and corrosion-resistant
Lid Design: Pressure-resistant vertical lid with radial locking arms, safety interlock mechanism, and silicone gasket
Sterilization Temperature: 121°C and 134°C cycle presets with ±1°C temperature accuracy
Operating Pressure: 0.22 MPa (2.2 bar) nominal operating pressure
Steam Source Options: Integrated electrical steam generator (standard) or external steam inlet (optional)
Control Interface: Microprocessor-based digital display / Touchscreen PLC with user-friendly navigation and multiple cycle storage
Cycle Types:
Wrapped/unwrapped solid instruments
Liquids in open containers
Porous load (optional vacuum pump required)
Glassware & media
Drying Function: Optional hot air or vacuum drying for enhanced instrument turnaround
Water Feed: Manual or automatic water filling (RO/DI compatibility supported)
Chamber Drainage: Thermostatically controlled with heat protection and antibacterial trap
Printer/Data Logging: Real-time printout or USB export for cycle validation and traceability

Manufactured under ISO 13485 and ISO 9001 Quality Systems
EN 13060 or EN 285 (depending on model class)
CE Marked – MDR Compliant
FDA 510(k) documentation package (for U.S. market)
IQ/OQ/PQ Validation Package available upon request

Let HEDONE USA become your OEM sterilizer manufacturing partner, offering U.S.-level engineering, global compliance, and industrial durability.
Contact us now to receive the technical dossier, MOQ, lead time, and price quotation.
Custom branding (logo, interface, panel design, color scheme)
Turnkey documentation for regulatory submission
Rapid lead times with short-run or high-volume manufacturing
Engineering support from prototype to scale
Spare parts availability & logistics coordination
U.S.-based representation and after-sales coordination